Lyman-alpha line
In physics, the Lyman-alpha line, sometimes written as Ly-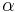 line, is a spectral line of hydrogen, or more generally of one-electron ions, in the Lyman series, emitted when the electron falls from the
line, is a spectral line of hydrogen, or more generally of one-electron ions, in the Lyman series, emitted when the electron falls from the 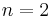 orbital to the
orbital to the 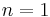 orbital, where n is the principal quantum number. In hydrogen, its wavelength of 1215.668 angstroms (1.216×10-7m), corresponding to a frequency of 2.47 × 1015 hertz, places the Lyman-alpha line in the vacuum ultraviolet part of the electromagnetic spectrum.
orbital, where n is the principal quantum number. In hydrogen, its wavelength of 1215.668 angstroms (1.216×10-7m), corresponding to a frequency of 2.47 × 1015 hertz, places the Lyman-alpha line in the vacuum ultraviolet part of the electromagnetic spectrum.
Because of fine structure perturbations, the Lyman-alpha line splits into a doublet. Specifically, because of the electron's spin-orbit interaction, the stationary eigenstates of the perturbed Hamiltonian must be labeled by the total angular momentum j of the electron (spin plus orbital), not just the orbital angular momentum 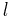 . In the
. In the 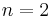 orbital, there are two possible states,
orbital, there are two possible states, 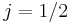 and
and 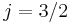 , resulting in a spectral doublet. The
, resulting in a spectral doublet. The 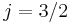 state is of higher energy (less negative) and so is energetically farther from the
state is of higher energy (less negative) and so is energetically farther from the 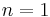 orbital to which it is transitioning. Thus, the
orbital to which it is transitioning. Thus, the 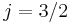 state is associated with the more energetic (shorter wavelength) spectral line in the doublet.
state is associated with the more energetic (shorter wavelength) spectral line in the doublet.
A K-alpha, or Kα, line analogous to the Lyman-alpha line for hydrogen, occurs in the high-energy induced emission spectra of all chemical elements, since it results from the same electron transition as in hydrogen. The equation for prediction of the frequency of this line (usually in the X-ray range for heavier elements), uses the same base-frequency as Lyman-alpha, but modified by a (Z-1)² factor to account for differing atomic numbers (Z) between elements, and is expressed as Moseley's law. The Lyman-alpha line and the rest of the hydrogen Lyman spectral series are most simply described by the empirical Rydberg equation and semi-classic Bohr model of the atom.